Please choose a body region on the right for you to pin point the problem area of your body.

Shop by Condition

Shop by Brand
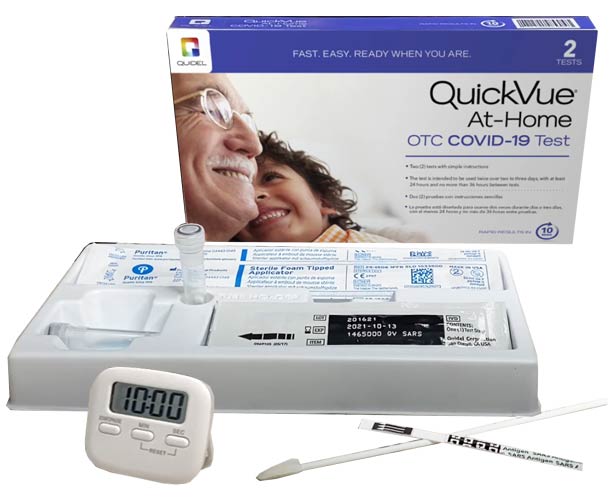
QuickVue COVID-19 Rapid Test - OTC Home Test Kit
Item # OTC-COVID 25 TESTS | SKU # QUI-20398
Test in the privacy of your home or where needed with our over the counter COVID-19 Test and get results in 10 minutes with our QuickVue At-Home OTC COVID-19 Test. It's fast, easy, and ready when you are!
Made by Quidel, everything you need is in the package and taking the test is simple. The test is authorized for home use with self-collected anterior nasal (nares) swab samples in individuals aged 2 and older.
Quickvue Rapid COVID test is also authorized for home use for individuals aged 2 through 14 with an adult performing the test. The test is intended to be used twice over two to three days, with at least 24 hours and no more than 36 hours between tests.
Our Quickvue OTC COVID-19 Test is authorized for nonprescription home use with self-collected (unobserved) direct anterior nasal (NS) swab specimens from individuals aged 14 years and older or with adult-collected anterior NS samples from individuals aged 2 years or older.
The Quickview COVID At-Home Test to be used in individuals within 6-days of symptom onset or individuals without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at least 24 hours (and no more than 48 hours) between tests.
Availability for the QuickVue COVID-19 Rapid Test - OTC Home Test Kit
| Model No. | Packaging | |
| 20398 | Bulk Kit - 25 Tests | 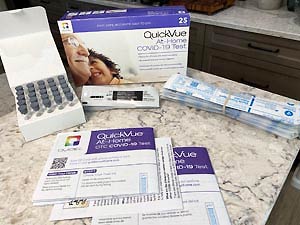 |
*Timer pictured above is not included.
This product has not been FDA cleared or approved; but has been authorized by FDA under an Emergency Use Authorization (EUA) This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
What is the history of the QuickVue® brand?
The QuickVue® brand launched in 1986 with visually read rapid diagnostics focusing on women’s health and respiratory diseases. In 1999, QuickVue® Influenza A+B was the first visually read rapid test approved by the FDA for professional use. QuickVue® At-Home OTC COVID-19 Test utilizes the same technology used for decades by healthcare professionals and by the QuickVue® SARS Antigen Test used in professional settings, receiving emergency use authorization (EUA) by the FDA in December 2020.
Has this product been approved by the FDA?
The QuickVue® At-Home OTC COVID-19 Test received Emergency Use Authorization (EUA) from the FDA for In Vitro diagnostic use only on March 31, 2021.
Has this product been approved for use in Canada?
The QuickVue® At-Home OTC COVID-19 Test has been approved for use in Canada per Health Canada Interim Order 331681.
What is an antigen test?
Antigen tests detect proteins of the SARS-CoV-2 virus that form during the infection cycle and indicate that a person has an active infection. Rapid antigen tests offer several important benefits. They are highly portable, fast, and easy to use, providing a flexible approach to helping more people get tested reliably, timely, frequently, and in a cost-effective way.
What is the accuracy of the test?
In a clinical study, the QuickVue® At-Home OTC COVID-19 Test identified positive cases 83.5% of the time, and identified negative cases 99.2% of the time (83.5% PPA, and 99.2% NPA, respectively), when compared to PCR.
How does the test work?
It’s as easy as Swab, Swirl, Dip and See Results. Following a gentle nasal swab sample, the QuickVue® At-Home OTC COVID-19 Test strip is mixed into the reagent solution where it rests for 10 minutes. After 10 minutes, the strip is removed, and individuals can review the results immediately.
How long does it take to get results?
Results are available in the privacy of your own home in as little as 10 minutes. Is a prescription needed to purchase the test? You do not need a doctor’s prescription to purchase and perform this test.
Is this test recommended for the asymptomatic consumer?
The QuickVue® At-Home OTC COVID-19 Test is intended for use in individuals with or without symptoms or other epidemiological reasons to suspect a COVID-19 infection.
Do the tests have an expiration date?
QuickVue® At-Home OTC COVID-19 Test has an 18-month shelf life. The expiration date will be clearly labeled on the test kit packaging.
Sizes 2 oz to 1000 mL available. Help Stop The Spread Of Germs with our Alcohol-based Purell Instant…
More Details >
Test in the privacy of your home or where needed with our over the counter COVID-19 Test and…
More Details >

Get $10 off your next order when you sign up to receive our email newsletter.*
Simply enter your email address below!
*Minimum order value of $100. Valid email address to qualify.